Mass Spectrometry analytical platform
The analytical platform is part of Team 3 (“Myelination and Nervous System Pathologies”) within UMRS1124 – HealthFex (Health and Functional Exposomics). It will be located on the 4th floor, rooms P463–P464. This mass spectrometry platform will enable comprehensive characterization of the entire steroidome from central and peripheral nervous system tissues, cells, biological fluids of animal or human origin, and whole organisms.
Scientific and Operational Manager
- Philippe LIERE, Research Engineer - INSERM, Mass Spectrometry analytical platform, Myelination and Nervous system pathologies
Activities
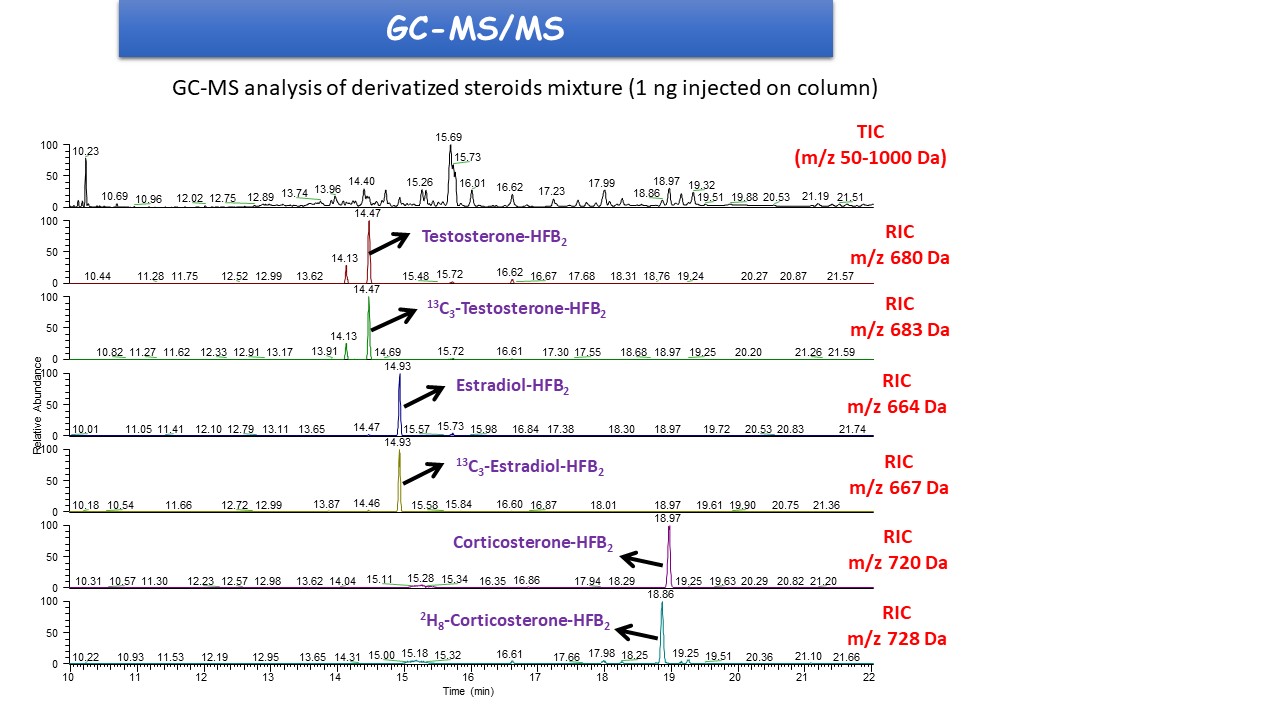
The analytical chemistry technology platform enables comprehensive steroidome characterization from tissues and biological fluids using state-of-the-art triple quadrupole tandem mass spectrometry coupled with high-resolution gas chromatography (GC-MS/MS).
This is currently the most sensitive (down to the femtogram level), selective, and accurate reference technology for mapping steroid profiles, allowing the analysis of up to 100 steroids, including progestagens, androgens, estrogens, gluco- and mineralocorticoids, steroid sulfates, oxysterols, sterols, phytosterols, vitamin D, as well as their precursors and metabolites.
The GC-MS/MS technology provides a three-dimensional analysis of molecules, allowing highly specific characterization of target compounds (Figure 1). Its specificity makes it possible to unambiguously identify structurally similar steroids, such as positional isomers and stereoisomers. The introduction of stable isotope-labeled steroids (13C or 2H) as internal standards ensures highly accurate quantification across the full range of target steroids.
The sample processing protocol including extraction, solid-phase extraction (SPE), high-performance liquid chromatography (HPLC) with fraction collection, chemical derivatization, and final GC-MS/MS analysis enables the detection of steroids with widely varying physicochemical properties, from the most hydrophilic (steroid sulfates) to the most hydrophobic (cholesterol, sterols) (Figure 2).
This analytical protocol has been validated for all complex biological matrices, such as tissues (a few mg) from the central and peripheral nervous systems (brain subregions, spinal cord, sciatic nerve), peripheral tissues (adrenal glands, testes, liver, lung, etc.), whole organisms (C. elegans, zebrafish), cells and their media, and all types of biological fluids (25–50 µL), such as plasma, saliva, cerebrospinal fluid, and urine, with very high reliability.
Our platform is unique and internationally recognized in the fields of endocrinology, neuroendocrinology, and neuroscience. It has contributed to major discoveries in various pathologies, both in animal models and in humans, and has opened new avenues of research.
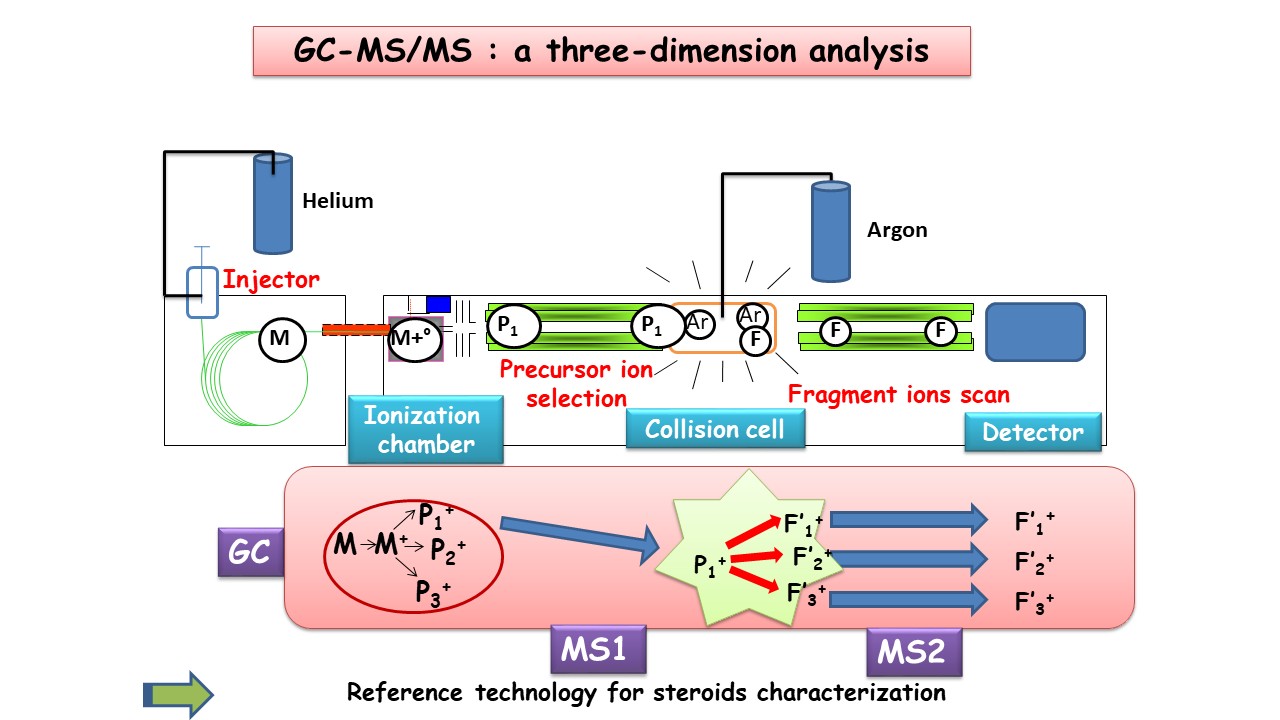
The GC-MS/MS technology provides a three-dimensional analysis of molecules, allowing highly specific characterization of target compounds (Figure 1). © all rights reserved
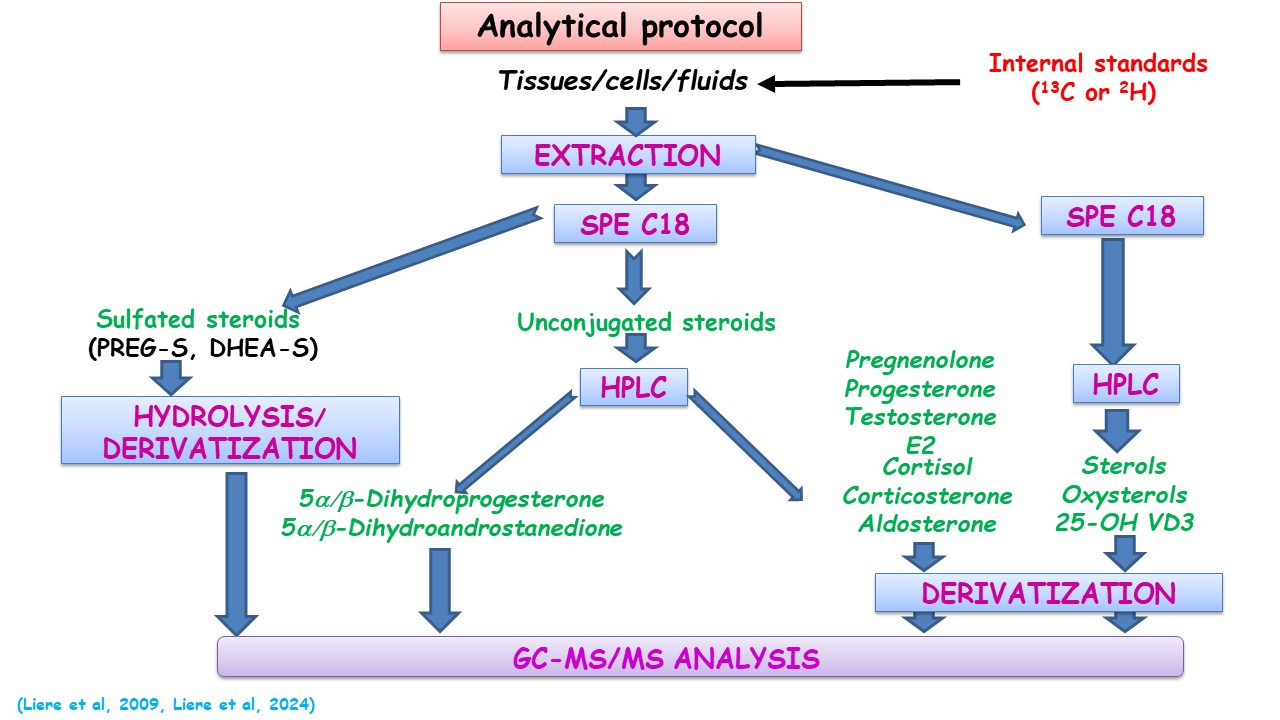
The sample processing protocol including extraction, solid-phase extraction (SPE), high-performance liquid chromatography (HPLC) with fraction collection, chemical derivatization, and final GC-MS/MS analysis enables the detection of steroids with widely varying physicochemical properties, from the most hydrophilic (steroid sulfates) to the most hydrophobic (cholesterol, sterols) (Figure 2). © all rights reserved
This technology has revolutionized the field of steroid neuroendocrinology and deepened our understanding of steroid biosynthesis in neural and peripheral tissues. It has revealed (neuro)endocrine dysregulations under specific pathophysiological conditions, identifying affected biosynthetic and signaling pathways (via PR, AR, ER, MR, GR, GABAAR) and their implications in neurodegenerative diseases, strokes, and nervous system injuries both in experimental models and in clinical research.
Our technology has also demonstrated the key role of sexual dimorphism in the central nervous system under various pathophysiological conditions (ischemia, brain trauma, stress responses) and in neurodegenerative diseases (Alzheimer’s, multiple sclerosis, Parkinson’s, etc.).
The GC-MS/MS analytical procedure has further enabled the identification of new steroid compounds in the rodent brain that had never been described in the literature. Technological developments within the platform have significantly improved sensitivity, now allowing longitudinal studies with micro blood sampling in murine models and salivary steroidome analysis in humans and large mammals.
Since GC-MS/MS technology can analyze non-thermolabile molecules with molecular weights below 1100 Da, it can also characterize compounds such as neurotransmitters (dopamine, serotonin, GABA, glutamate, etc.), endocrine disruptors (phytoestrogens, bisphenols, phthalates, PCBs, dioxins, PAHs), persistent organic pollutants (PFAS), and thyroid hormones.
Equipment
The technological platform will be housed in a climate-controlled laboratory (20 ± 2 °C) with controlled humidity (40–60%). It will be equipped with a chemical fume hood, an ultracentrifuge, a sonication bath, and a nitrogen evaporator–concentrator for extraction and purification steps.
The platform will also include a Solid Phase Extraction (SPE) system (Vac Elut SPS 24, Agilent Technologies) (Figure 3), which purifies biological extracts through selective adsorption of steroids based on their physicochemical properties on a hydrophobic solid phase. This step removes most hydrophilic and hydrophobic compounds while isolating the most hydrophilic (steroid sulfates) and non-conjugated steroids (such as steroid hormones) (Figure 4).
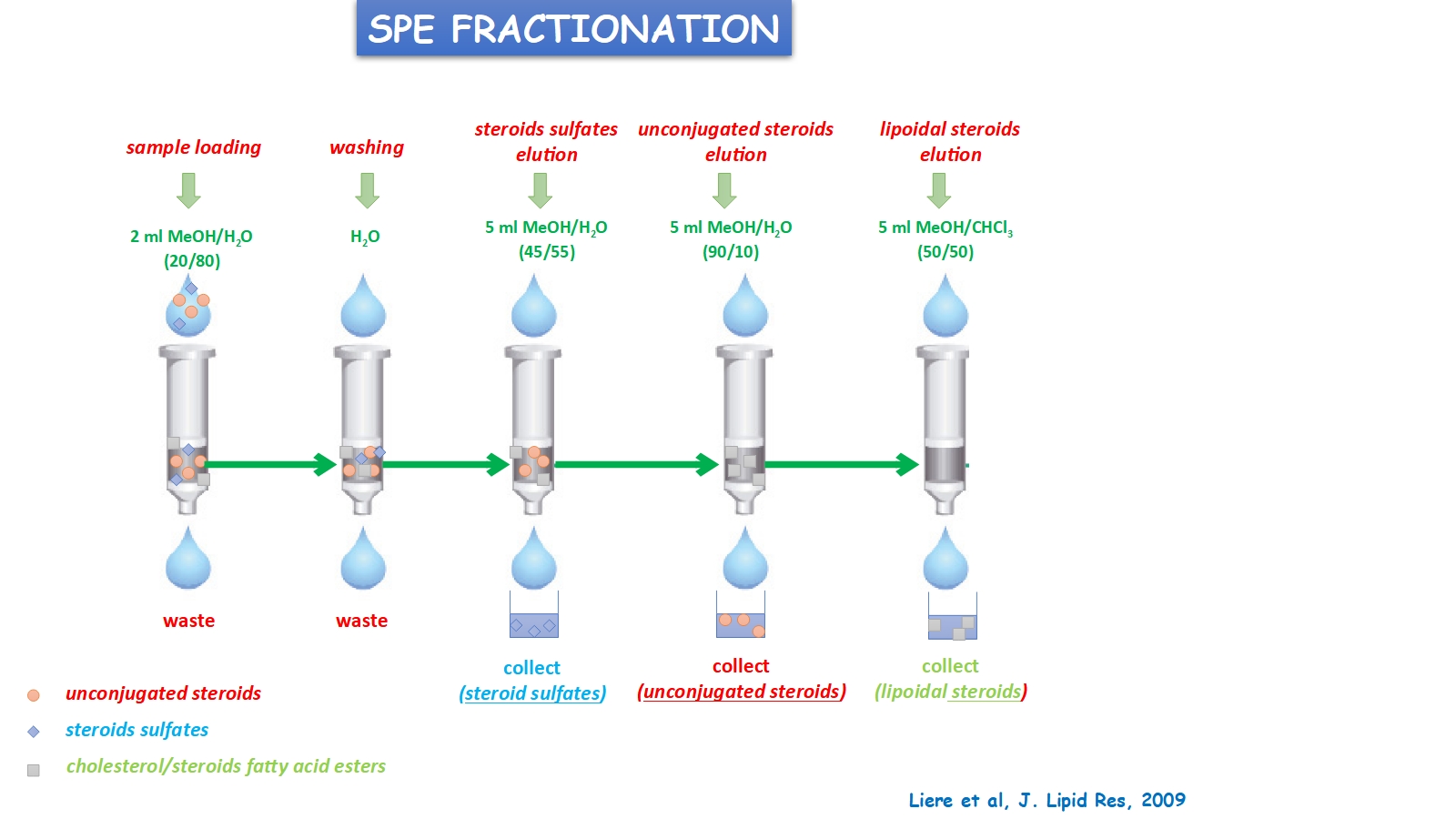
This step removes most hydrophilic and hydrophobic compounds while isolating the most hydrophilic (steroid sulfates) and non-conjugated steroids (such as steroid hormones) (Figure 4) © all rights reserved

a Solid Phase Extraction (SPE) system (Vac Elut SPS 24, Agilent Technologies) (Figure 3) © all rights reserved
The fraction containing non-conjugated steroids is further filtered, purified and fractionated by High-Performance Liquid Chromatography (HPLC) with a fraction collector (Vanquish Core Quaternary Gradient HPLC, Thermo Fisher Scientific) (Figure 5).
This step separates more apolar ketonic steroids from more polar hydroxylated steroids, as the subsequent chemical derivatization step is structure-specific. Steroids from the isolated biological extract fractions are then chemically derivatized under precisely controlled conditions in dry baths at set temperatures, evaporated, and reconcentrated under nitrogen (Reacti-Therm III/Reacti-Vap Evaporator, Fisher Scientific) (Figure 6).

High-Performance Liquid Chromatography (HPLC) with a fraction collector (Vanquish Core Quaternary Gradient HPLC, Thermo Fisher Scientific) (Figure 5) © all rights reserved

Reacti-Therm III/Reacti-Vap Evaporator, Fisher Scientific (Figure 6) © all rights reserved
The analysis of biological extracts will be performed using GC-MS/MS (Trace 1300 Gas Chromatograph and TSQ8000 Mass Spectrometer, Thermo Fisher Scientific) (Figure 7).
A GC-MS instrument (Focus Gas Chromatograph and DSQII Mass Spectrometer, Thermo Fisher Scientific) will also be available for method development and optimization.
Data acquisition and processing (identification/quantification) are carried out using Xcalibur software (v4.6, Thermo Fisher Scientific) (Figure 8).
Main Publications
– Luchetti S, Mason MRJ, Liere P, Pianos A, Cossu S, Hofstee M, Sluiter A, Adelia, van Scheppingen J, Aronica E, Schumacher M, Huitinga I. 2025. Multiple sclerosis lesions exhibit sex-specific steroid changes. Neurobiol. Dis. 214:107040.
– Luchetti S, Liere P, Pianos A, Verwer RWH, Sluiter A, Huitinga I, Schumacher M, Swaab DF, Mason MRJ. 2023. Disease stage-dependent changes in brain levels and neuroprotective effects of neuroactive steroids in Parkinson’s disease. Neurobiol. Dis. 183:106169.
– Liere P, Liu GJ, Pianos A, Middleton RJ, Banati RB, Akwa Y. 2023. The Comprehensive Steroidome in Complete TSPO/PBR Knockout Mice under Basal Conditions. International J. Molecular Sciences. 24, 2474.
– Fernandez N, Petit A, Pianos A, Haddad L, Schumacher M, Liere P, Guennoun R. 2023. Aging Is Associated With Lower Neuroactive Steroids and Worsened Outcomes Following Cerebral Ischemia in Male Mice. Endocrinology.164, 1–24.
– Goudet G, Douet C, Pianos A, Haddad L, , Reigner F, Deleuze S, Liere P. 2022. Saliva and plasma steroidome in mare during reproductive stages: A GC-MS/MS study. Frontiers in Animal Science. 3, 10.3389/fanim.1055179.hal-03841907.
– Vacher CM, O’Reilly JJ, Salzbank J, Lacaille H, Bakalar D, Sebaoui-Illoul S, Liere P, Clarkson-Paredes C, Sasaki T, Sathyanesan A, Imamura Kawasawa Y, Popratiloff A, Hashimoto-Torii K, Gallo V, Schumacher M, Penn AA. 2021. Placental neurosteroids shape cerebellar development and social behavior. Nature Neuroscience. 24, 1392-1401.
– Liere P, Cornil CA, de Bournonville MP, Pianos A, Keller M, Schumacher M, Balthazart J. 2019. Steroid profiles in quail brain and serum: Sex and regional differences and effects of castration with steroid replacement. J Neuroendocrinol. Feb;31(2):e12681
– Berkane N, Liere P, Lefevre G, Alfaidy N, Nahed RA, Vincent J, Oudinet JP, Pianos A, Cambourg A, Rozenberg P, Galichon P, Rousseau A, Simon T, Schumacher M, Chabbert-Buffet N, Hertig A. 2018. Abnormal steroidogenesis and aromatase activity in preeclampsia. Placenta. Sep;69:40-49.
– Guennoun R, Fréchou M, Gaignard P, Liere P, Slama A, Schumacher M, Denier C, Mattern C. 2019. Intranasal administration of progesterone: A potential efficient route of delivery for cerebroprotection after acute brain injuries. Neuropharmacology. Feb;145(Pt B):283-291.
– Zhu X, Fréchou M, Liere P, Zhang S, Pianos A, Fernandez N, Denier C, Mattern C, Schumacher M, Guennoun R. 2017. A Role of Endogenous Progesterone in Stroke Cerebroprotection Revealed by the Neural-Specific Deletion of Its Intracellular Receptors. J Neurosci. Nov 8;37(45):10998-11020.
– Liere P, Pianos A, Oudinet JP, Schumacher M, Akwa Y. 2017. Differential effects of the 18-kDa translocator protein (TSPO) ligand etifoxine on steroidogenesis in rat brain, plasma and steroidogenic glands: Pharmacodynamic studies. Psychoneuroendocrinology. 83:122134.
– Gonzalez Deniselle MC, Liere P, Pianos A, Meyer M, Aprahamian F, Cambourg A, Di Giorgio NP, Schumacher M, De Nicola AF, Guennoun R. 2016. Steroid Profiling in Male Wobbler Mouse, a Model of Amyotrophic Lateral Sclerosis. Endocrinology. 157(11):4446-4460.
– Liere P, Schumacher M. 2015. Mass spectrometric analysis of steroids: all that glitters is not gold. Expert Rev Endocrinol Metab 10:463-465.
– Schumacher M, Guennoun R, Mattern C, Oudinet JP, Labombarda F, De Nicola AF, Liere P. 2015. Analytical challenges for measuring steroid responses to stress, neurodegeneration and injury in the central nervous system. Steroids. 103/42-57. Review
– Liu A, Margaill I, Zhang S, Labombarda F, Coqueran B, Delespierre B, Liere P, Marchand-Leroux C, O’Malley BW, Lydon JP, De Nicola AF, Sitruk-Ware R, Mattern C, Plotkine M, Schumacher M, Guennoun R. 2012. Progesterone receptors: a key for neuroprotection in experimental stroke. Endocrinology. 153(8):3747-57.
– Hertig A, Liere P, 2010. New markers in preeclampsia. Clin Chim. Acta, 411, 1591-1595.
– Hertig A, Liere P, Chabbert-Buffet N, Fort J, Pianos A, Eychenne B, Cambourg A, Schumacher M, Berkane N, Lefevre G, Uzan S, Rondeau E, Rozenberg P, Rafestin-Oblin ME. 2010. Steroid profiling in preeclamptic women:evidence for aromatase deficiency. Am. J. Obstet. Gynecol., 203(5): 03(5):477.e1-9.
– Liere P, Pianos A, Eychenne B, Cambourg A, Bodin K, Griffiths W, Schumacher M, Baulieu EE, Sjövall J. 2009. Analysis of pregnenolone and dehydroepiandrosterone in rodent brain: cholesterol autoxidation is the key. J Lipid Res. 50(12):2430-44.
– Schumacher M, Liere P, Akwa Y, Rajkowski K, Griffiths W, Bodin K, Sjövall J, Baulieu EE. 2008. Pregnenolone sulfate in the brain: a controversial neurosteroid. Neurochem Int. 52(4-5):522-40.
– Meffre D, Pianos A, Liere P, Eychenne B, Cambourg A, Schumacher M, Stein DG, Guennoun R. 2007. Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: analysis by gas chromatography/mass spectrometry. Endocrinology. 148(5):2505-17.
– Broué F, Liere P, Kenyon C, Baulieu EE. 2007. A steroid hormone that extends the lifespan of Caenorhabditis elegans. Aging Cell. 6(1):87-94.
– Liere, P., A. Pianos, B. Eychenne, A. Cambourg, S. Liu, W. Griffiths, M. Schumacher, J. Sjovall and E. E. Baulieu. 2004. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J Lipid Res., 45, 2287-2302.
– Weill-Engerer, S., J. P. David, V. Sazdovitch, P. Liere, B. Eychenne, A. Pianos, M. Schumacher, A. Delacourte, E. E. Baulieu and Y. Akwa. 2002. Neurosteroid quantification in human brain regions: comparison between Alzheimer’s and nondemented patients. J Clin Endocrinol Metab. 87: 5138-43.
– P. Liere, Y. Akwa, S. Weill-Engerer, B. Eychenne, A. Pianos, P. Robel, J. Sjövall, M. Schumacher and E.E. Baulieu, 2000, Validation of an analytical procedure to measure trace amounts of neurosteroids in brain tissue by gas chromatography-mass spectrometry, Journal of Chromatography B., 739, 301.